Infertility affects one in seven men of reproductive age worldwide. One idea for treating male sterility is spermatogonial stem cell (SSC) therapy. In this approach, sperm stem cells in the testis are transferred to a test tube, cultured and nudged into becoming fully fledged sperm. However, a key bottleneck has been identifying just the right conditions to get human SSCs to grow in the lab. There have been many attempts, but in most reported cases it was not clear whether the cells being cultured were actually SSCs, and no previously published method is routinely used.
Researchers at University of California San Diego School of Medicine have now developed a reliable method for culturing cells with the characteristics of human SSCs. Their work is published in the July 13, 2020 issue of Proceedings of the National Academy of Sciences.
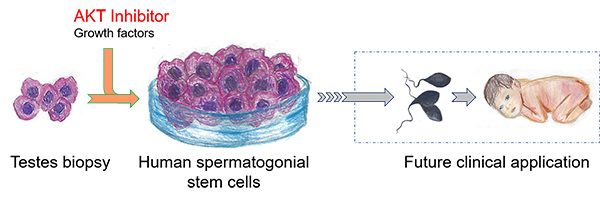
By applying an inhibitor of the molecule AKT, UC San Diego researchers are able to favor the culture of human spermatogonial stem cells in the lab, a first step toward lab-produced sperm as a treatment for male infertility. Illustrations by Vishaala Wilkinson
“We think our approach — which is backed up by several techniques, including single-cell RNA-sequencing analysis — is a significant step toward bringing SSC therapy into the clinic,” said senior author Miles Wilkinson, PhD, Distinguished Professor in the Department of Obstetrics, Gynecology and Reproductive Sciences at UC San Diego School of Medicine.
SSCs are what make it possible for men to father children beyond the age of 65. These specialized cells continually self-renew, making more SSCs, and develop into sperm so prolifically that men (and some transgender, non-binary and gender fluid people) produce more than 1,000 new sperm every few seconds.
Progress in the field has been hindered by the fact that it’s extremely difficult to distinguish SSCs from other cells in the testes. It was a major step forward when several laboratories, including the Wilkinson team, recently used a technique called single-cell RNA sequencing to define the likely molecular characteristics specific to human SSCs.
In their latest effort, the Wilkinson team used its single-cell RNA sequencing information to purify what it thought might be human SSCs. Using a method called germ-cell transplantation, it showed that the cells it purified were indeed highly enriched in human SSCs. The team then gathered the profile of genes expressed in these human SSCs to make guesses as to the conditions that might best support their growth in the lab. Using more than 30 human testis biopsies, the researchers determined just the right conditions needed to culture immature germ cells with the characteristics of SSCs.
The key ingredient was an inhibitor of the AKT pathway, a cellular system that controls cell division and survival. The Wilkinson team determined that AKT inhibition maintains human SSCs by inhibiting development of later-stage sperm precursors. Several AKT inhibitors are currently used to treat cancer.
With that approach, the researchers were able to favor the culture of human cells with the molecular characteristics of SSCs for two-to-four weeks.
“Next, our main goal is to learn how to maintain and expand human SSCs longer so they might be clinically useful,” Wilkinson said.
Co-authors of the study include: Kun Tan, Hye-Won Song, Merlin Thompson, Tung-Chin Hsieh, UC San Diego; Sarah Munyoki, Meena Sukhwani, and Kyle E. Orwig, University of Pittsburgh School of Medicine and Magee-Womens Research Institute.
Funding for this research came, in part, from the National Institutes of Health (grants T32-HD087194, R01-HD092084 and R01-GM119128) and Lalor Foundation.
UC San Diego
