Oxford and AstraZeneca’s COVID vaccine trial is put on HOLD as British volunteer has ‘serious’ reaction ‘that triggered spinal cord infection’
- Worldwide trials of jab paused indefinitely after British patient rushed to hospital
- Thought to have suffered serious spinal cord infection, according to sources
- Investigation launched to tell if vaccine caused the side effect or coincidence
Trials of Oxford University’s coronavirus vaccine have been put on hold after a British volunteer was rushed to hospital with a suspected spinal cord infection thought to be triggered by a reaction to the jab.
UK drug giant AstraZeneca, which owns the rights to the vaccine, said all studies of the jab had been paused indefinitely while it investigates whether the patient’s side-effect is connected to the vaccine.
AstraZeneca did not reveal any information about the volunteer’s condition other than to say they had suffered a ‘serious adverse reaction’.
It’s unclear what the exact nature of the reaction was, but the New York Times quotes a source claiming the patient had transverse myelitis, an inflammation of the spinal cord that can cause permanent paralysis in some patients.
The disorder can be triggered from a number of causes that set off the body’s inflammatory responses, including viral infections.
The development is a major blow to worldwide hopes for a jab to be ready by Christmas because the Oxford vaccine was considered by many – including the World Health Organization – to be the frontrunner.
Health Secretary Matt Hancock said the pause to the Oxford vaccine trial is not necessarily a setback and that trials of the jab were put on hold over summer when a patient fell ill.
He told Sky News: ‘It is obviously a challenge to this particular vaccine. It’s not actually the first time it has happened to the Oxford vaccine and it’s a standard process in clinical trials.’
Asked if it will push back the rollout of the vaccine, Mr Hancock said: ‘Not necessarily – it depends on what they find when they do the investigation. There was a pause earlier in the summer and that was resolved without a problem.’

Trials for AstraZeneca’s shot are underway in the US, UK, Australia, Brazil (pictured) and other nations. Phase 3 testing will now be paused while safety data is reviewed
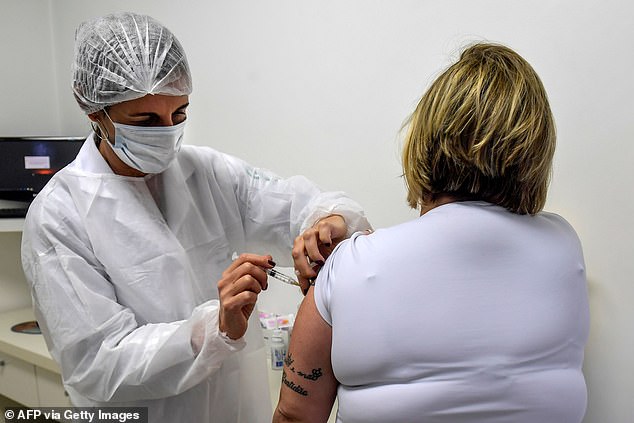
AstraZeneca’s candidate vaccine, known as AZD1222, is in phase 3 trials – the final stage before safety and efficacy data can be submitted to regulators. Pictured: A Brazilian volunteer receiving the Oxford vaccine, July 24
Professor Terry Nolan from the University of Melbourne, said it was entirely plausible the volunteer had suffered transverse myelitis (TM) as a direct result of the vaccine.
The exact cause of TM is unknown, but it has been reported to occur after infections and vaccinations.
News site Stat first reported the pause in testing and said the possible side-effect occurred in a testing volunteer in Britain, who was expected to recover.
The vaccine, developed by Oxford University, is being tested in thousands of people in Britain and the US, and in smaller study groups in Brazil and South America.
An AstraZeneca spokeswoman said the pause is part of a standard review process which occurs in trial if there is a ‘potentially unexplained illness’ reported in any trial subject, and that the subject’s illness could also be coincidental.
‘As part of the ongoing randomised, controlled global trials of the Oxford coronavirus vaccine, our standard review process was triggered and we voluntarily paused vaccination to allow review of safety data by an independent committee,’ the spokeswoman said in a statement.
‘This is a routine action which has to happen whenever there is a potentially unexplained illness in one of the trials, while it is investigated, ensuring we maintain the integrity of the trials.
‘In large trials illnesses will happen by chance but must be independently reviewed to check this carefully.
‘We are working to expedite the review of the single event to minimise any potential impact on the trial timeline. We are committed to the safety of our participants and the highest standards of conduct in our trials.’
No details about the patient suffering the potential side-effect, or the nature of the reaction, were given.
Temporary holds of large medical studies are not uncommon, and looking into any unexpected reactions is a mandatory part of safety testing. It was not immediately clear how long AstraZeneca’s pause would last.
Two other vaccines are in huge, final-stage tests in the United States, one made by Moderna Inc and the other by Pfizer and Germany’s BioNTech.
Stats reported a total of nine vaccine candidates in late stage, or phase 3, trials, with AstraZeneca’s the first trial known to have been put on hold.
Despite some figures, such as US President Donald Trump, insisting a vaccine will be ready in a matter of months, Oxford University has said a vaccine might not be ready before 2022.
The university stressed that clinical trials have to be conducted with the utmost care.
‘It takes time to develop safe and effective vaccines – usually five to 10 years on average. Despite promising reports about potential coronavirus vaccines being developed worldwide, it could still take an estimated 12-18 months to develop one,’ a document on the university’s website, dated August 25, reads.
‘It is essential that clinical trials are conducted with great care to ensure the safety of the participants and to fully establish the safety profile of the new products.
‘Safety is overseen closely during the trials both by the national regulator with a requirement of safety reporting placed on investigators throughout the trial, and inspections of the trial processes and procedures by the regulator, and an independent safety monitoring committee who reviews safety actively during the conduct of the clinical trial.
‘When an application for use of the vaccine is made to a regulator, they will fully assess the safety and efficacy data from the trials and use that to inform on their decision about potential use.’
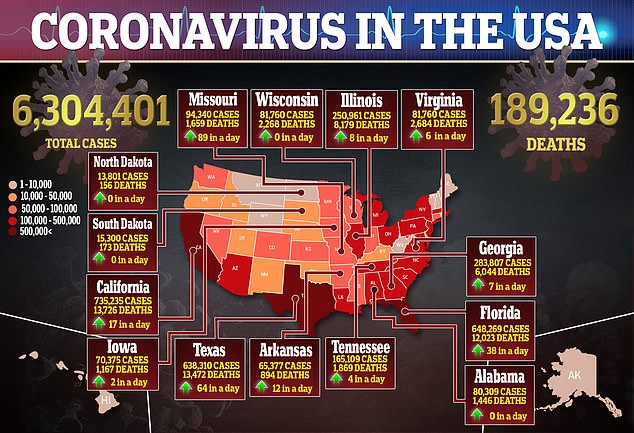
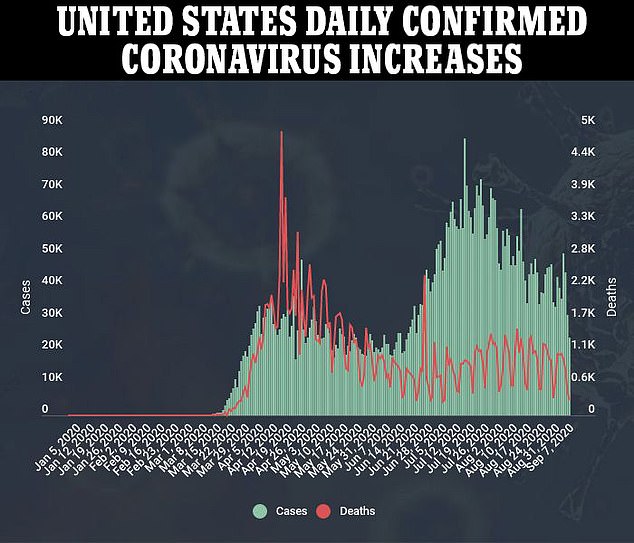
Development of the AstraZeneca vaccine and eight others in phase 3 trials is being closely watched in the hopes they can stem the coronavirus pandemic that has killed more than 894,000 people worldwide, including nearly 190,000 Americans, and cost tens of millions their jobs.
It comes after vaccine developers – including AstraZeneca – pledged not to cut corners on safety and efficacy testing, despite US President Trump’s urgent push for the Food and Drug Administration (FDA) to give emergency approval to a vaccine ahead of the November 3 election.
‘As part of the ongoing randomised, controlled globa
l trials of the Oxford coronavirus vaccine, our standard review process was triggered and we voluntarily paused vaccination to allow review of safety data by an independent committee,’ an AstraZeneca spokesperson told DailyMail.com.
‘This is a routine action which has to happen whenever there is a potentially unexplained illness in one of the trials, while it is investigated, ensuring we maintain the integrity of the trials.
‘In large trials, illnesses will happen by chance but must be independently reviewed to check this carefully. We are working to expedite the review of the single event to minimise any potential impact on the trial timeline. We are committed to the safety of our participants and the highest standards of conduct in our trials.’
AstraZeneca’s candidate vaccine, known as AZD1222, is in phase 3 trials – the final stage before safety and efficacy data can be submitted to regulators for approval – at dozens of sites across the US, and around the world.
Along with Pfizer, and Moderna, AstraZeneca and its partner Oxford University had hoped to know whether the shot worked and was safe by year-end.
Mr Hancock said on Monday the UK’s ‘best-case scenario’ was to get the vaccine to the most-vulnerable patients within months, a timeframe that now appears less likely to be met.
‘We have got 30 million doses already contracted with AstraZeneca,’ he said on UK radio station LBC.
‘In fact they are starting to manufacture those doses already, ahead of approval, so that should approval come through – and it’s still not certain but it is looking up – should that approval come through then we are ready to roll out.
‘The best-case scenario is that happens this year. I think more likely is the early part of next year – in the first few months of next year is the most likely.
‘But we’ve also bought vaccine ahead of it getting approved from a whole different series of international vaccines as well.’
More than 50,000 people worldwide had been taking part in ‘phase 3’ studies to see whether the Oxford jab can actually prevent people getting infected with Covid-19.
In these tests the vaccine is being given to tens of thousands of people in real-world environments to see if it stops them from catching Covid-19 in the community.
While trial holds to review safety data are not necessarily damning, the pause on AstraZeneca’s trial may very well delay the highly-anticipated results and completion of one of the fastest vaccine development pipelines in human history.

The jab was expected at the end of 2020 but its creators have had to temper expectations after community transmission began to fizzle out in Britain and stall crucial trials needed to seal its approval. It was called ChAdOx1 nCoV-19 before Oxford teamed up with AstraZeneca to manufacture it

Shares for AstraZeneca plummeted by eight percent in after-hours trading
Market confidence in the pharmaceutical giant took a hit as soon as reports of the trial hold emerged.
Shares for AstraZeneca plummeted by eight percent in after-hours trading.

President Donald Trump has hinted that he thinks a vaccine could be ready before a ‘special date’ – likely the November 3 election
The company is in now in mitigation mode to try to stay as close to its trial completion date as possible.
AstraZeneca is ‘working to expedite the review of the single event to minimize any potential impact on the trial timeline,’ the spokesperson told Stat News.
Disconcerting though any signs that could suggest potential flaws in a leading vaccine for the disease that’s killed nearly 190,000 Americans is, the move to pause a trial over safety concerns may be reassuring to some.
Hours before the trial was temporarily halted, AstraZeneca and eight other companies working on COVID-19 vaccines signed a pledge to prioritize safety of their shots over speedy development.
They promised to ‘uphold the integrity of the scientific process as they work towards potential global regulatory filings and approvals of the first COVID-19 vaccine.’
The pledge was made in response to growing concerns that governments would press firms and research institutions to rush a vaccine through trials and approval processes in an effort to bolster political capital and restore normalcy amid the pandemic.
Last week, the US Centers for Disease Control and Prevention (CDC) sent instructions to state health departments to prepare for the potential arrival of one of two coronavirus vaccines by the end of October.
Subsequently, President Trump hinted at the hopeful possibility that a vaccine could be ready before a ‘special date,’ prompting speculation that he was referring to the November 3 presidential election – just days after the end-of-October potential finish line in the CDC guidance.
Health officials like infectious disease exp
ert Dr Anthony Fauci, US Surgeon General Dr Jerome Adams and Trump’s own vaccine czar Dr Moncef Slaoui were all quick to assure the American public that it was possible, but unlikely, that a shot would be ready by then, and that the FDA would pay no mind to the date, but only the data, to determine when a vaccine is ready for distribution.
Pfizer, however, confirmed that it hoped to have sufficient trial data to know whether its shot was safe and effective by late October, and would submit for FDA approval ‘immediately’ afterwards.
Most experts and observers speculate that the two unnamed shots referenced in the CDC guidance were Moderna’s and Pfizer’s, not AstraZeneca.
The trial hold casts even further doubt over the possibility that the firm’s vaccine will be the first ready for the US market.
Operation Warp Speed, the Trump administration’s effort to speed vaccine development, primarily by contributing funding to private companies working on shots, announced that AstraZeneca would be awarded up to $ 1.2 billion in funding for its vaccine development in May.
The US has inked a deal with AstraZeneca for 300 million doses of its shot – if the vaccine is greenlit as safe and effective by the FDA – and had set its sights on an approval date as early as October, which seems increasingly improbable.
